Family Muricidae (muricids) is a diverse family of marine snails with about 1,600 living species and about 1,200 recognized fossil species. They are voracious predators, and their shells range in size from small to large (up to 30.5 cm in length). Their shells are characterized by commonly having at least three varices (prominent longitudinal ridges indicating temporary stoppage of the growth of the shell).
Muricids can secrete a purple dye, which is used in ancient human cultures in the making of the "royal Tyrian purple," which is used to stain ceremonial robes. This dye, which is used by these snails for predation purposes, is toxic to crabs and fish. In a future post, I shall go into more detail about this interesting subject.
Many muricid species inhabit rocky or rubble bottoms, but many live on muddy bottoms, especially those living in depths greater than 200 m.
Based on the genera Morea and Sargana, the fossil record of muricids (most likely extends as far back as near the end of the Late Cretaceous The earliest confirmed muricids (e.g., Poirieria, Paziella, Pterynotus) are of Paleocene age.
The following series of five groups (A-E) shows images of the front and back views of five well-known examples of muricid shells. Each pertinent caption precedes its respective couplet of images.
(A) Siratus alabaster (Reeve, 1845) is known from SE Japan, Taiwan, and the Philippines. This species lives in deep water and is locally common. Its shell is ivory white, up to 20 cm height, and has three winged (alate = weblike) varices. Including the spines, this specimen has a height of 13.5 cm and a width of 10 cm.
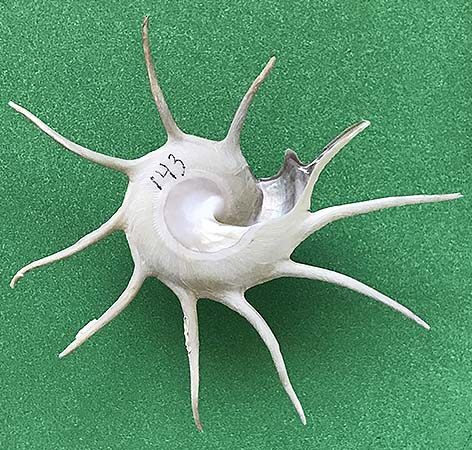
(B) Murex pecten Lightfoot, 1786, famously known as the “Venus comb,” is known from Japan to Queensland, Australia, and the Solomon Islands in the Pacific Ocean. The shell of this muricid excessively sharp spines on its three varices. Emanating from the lateral varices on each side of its shell is a row of spines that curve downward, thereby causing the shell to be elevated above the ocean floor and creating a protected area when the head of the gastropod extends outward in order to feed. Including the spines, this shell has a height of 13.5 cm, and a width of 8 cm.
C) Murex tribulus Linnaeus, 1758 is known from the entire Indo-Pacific region [from SE Japan to NE Australia and E Africa). Including its spines, this shell has a height of 10.5 cm and a width of 6 cm.
D) Chicoreus cervicornis (Lamarck, 1822) is known from Queensland, Australia and north and west to northwestern Australia. This shell has a height of 6.5 cm and a width of 5.5 cm.
E) Homalocantha zamboi (Burch and Burch, 1960), is known from the Philippines and the Solomon Islands in the Pacific Ocean. The illustrated specimen, which has a height of 5.5 cm and a width of 5 cm, has five varices.
References Consulted:
Radwin, G.E. and A.D’Attilio. 1976. Murex shells of the world. An illustrated guide to the Muricidae. Stanford University Press, Stanford, California. 284 pp.
WoRMS (2023)